Matthew's Science E-Portfolio
Saturday, 15 September 2012
Science - Overview: Personal Reflection
Personally, I feel that this year's Science has been hard to master, but it has been enjoyable to do so.
Secondary 2 Science comprised of 3 different topics, Chemistry, Physics and Biology, which could be split up into many different sub-topics. Thus, it was very hard to successfully grasp all the concepts during the time frame of 9 months, combined with all the other subjects.
In my opinion, Biology was the hardest topic to master this year, as Biology had many technical terms that we had to clarify with the Science teacher multiple times, but I failed to do so. As a result, I did not do so well for Biology in term 3. However, I still feel that Science is a very interesting subject.
Science Grades:
Term 1- B4(27.5/45)
Could have done better, but I didn't study. Displeased with results :<
Term 2- A2 (32/45)
Woke up this term, and decided to pay attention in class a bit more. With my classmate's help, Matthew Lim, I managed to score an A2 for Science :D Yay!
Term 3- C6(24/45)
Slacked this term, didn't study at all, payed very little attention in class. I deserve my term 3 results and intend to work very hard to do better in term 4.
Target for Term 4 Science Grade: A1
Science Project ACE for Term 2: http://db.tt/NdKM5P2h
Salt Preparation
So what is a salt?
A salt is the ionic compound formed when a metallic or ammonium ion partially or completely replaces the hydrogen ions of an acid. It contains a positive metal (or ammonium) ion from a base and a negative non-metal ion from an acid.
e.g. Ca(OH)2 + H2SO4 -> CaSO4 + 2H2O
Water of Crystallization
- Salt crystals are formed when salts combine with water molecules. These water molecules are known as water of crystallization.
- Salts that contain water of crystallization are called hydrated salts.
- Water of crystallization can be removed from a salt when the hydrated salt is heated, leaving behind an anhydrous form of the salt.
- Anhydrous salts refer to salts that do not contain water of crystallization. They are often in the form of powders.
Solubility of Salts
Although salts are ionic compounds, not all salts are soluble in water. All Sodium, Potassium, Ammonium and Nitrate salts are soluble, all Chloride, Bromide and Iodide salts are soluble except for Silver and Lead (II) ones, all Sulfate salts are soluble except for Barium, Calcium and Lead (II) ones, all Carbonates are insoluble except for Sodium Carbonate, Potassium Carbonate and Ammonium Carbonate, and all Oxides and Hydroxides are insoluble except for Sodium, Potassium and Barium ones.
How to form insoluble salts
All insoluble salts can be prepared by precipitation. To precipitate an insoluble salt, mix solution A which contains the positive ions (cations) of the salt, and solution B which contains the negative ions (anions) of the salt. For example, Lead (II) Sulfate can be prepared using a soluble Lead (II) salt (such as Lead (II) Nitrate) and dilute sulfuric acid or any soluble sulfate.
Acids, Bases and Writing Chemical Equations
Definition of an acid:
An acid is a substance which produces hydrogen ions as the the only positive ions when it is dissolved in water. Acids are either classified as weak or strong.
Some common strong acids are:
Hydrochloric acid (HCl)
Nitric Acid (HNO3)
Nitric Acid (HNO3)
Sulfuric Acid (H2SO4)
A common weak acid is Ethanoic acid, or CH3COOH.
Weak acids dissociate partially in water but a strong acids dissociates completely in water.
Properties of Acids
An acid, when dissolved in water, forms a colorless solution. Solutions of acids have:
- A sour taste
- Change the color of pH indicators (e.g. Turns blue litmus paper red)
- React with hydrogen ions and conduct electricity
- React with metals, carbonates and bases.
Acid + Metal -> Salt + H2
e.g. 2HCl + Zn -> ZnCl2 + H2
e.g. H2SO4 + CaCO3 -> CaSO4 + CO2 + H2O
Acid + Base -> Salt + H2O
e.g. MgO + H2SO4 -> MgSO4 + H2O
Properties of bases
Bases are the oxides or hydroxides of metal that reacts with an acid to form a salt and water only. Most bases are insoluble in water, and the bases that are soluble in water are called alkalis. These alkalis produce hydroxide ions (OH-) in water. All alkalis are bases but not all bases are alkalis.
Alkalis have:
- A soapy feel and a bitter taste
- Can change the color of pH indicators (turn red litmus paper blue)
- Can react with acids
- Can react with ammonium salts to form salt, ammonia and water
Base + Ammonium Salt -> Salt + NH3 + H2O
e.g. NaOH + NH4Cl -> NaCl + NH3 + H2O
- Can react with a solution of one metal salt to give metal hydroxide and another metal salt
Base + Metal Salt -> Metal Hydroxide + Another metal salt
e.g. CuSO4 + 2NaOH -> Cu(OH)2 + Na2SO4
e.g. CuSO4 + 2NaOH -> Cu(OH)2 + Na2SO4
Types of oxides:
- Acidic Oxide
- Basic Oxide
- Neutral Oxide
- Amphoteric Oxide
Acidic Oxide
An acidic oxide is a non-metallic oxide that neutralizes a base to form salt and water only. It reacts with water slightly to form an acid solution.
Basic Oxide
A basic oxide is a metallic oxide that neutralises with an acid to form salt and water only. If it is soluble in water, it forms an alkali.
Neutral Oxide
Neutral oxides are non-metallic oxides that have neither acidic nor basic properties. They are insoluble in water and do not react with water.
Amphoteric Oxide
Amphoteric oxides are metallic oxides that react with both acid and base to form salt and water. They have both acidic and basic properties.
Alkalis can be weak or strong. When strong alkalis dissolve in water, they become OH- ions in the solution.
Strong alkalis dissociates completely in water while weak alkalis dissociate partially in water.
Friday, 14 September 2012
Atomic Bonding
Why do atoms bond?
Noble gases such as helium, neon and argon are monoatomic because their valence shells are fully occupied by electrons. Thus, noble gases are stable and do not need to undergo bonding with other atoms.
Since only atoms with electronic configurations of noble gases are stable, then atoms bond to achieve the electronic configuration of a noble gas; or a fully filled valence shell. Atoms do so by transfer or sharing or electrons with other atoms.
By having an electronic configuration of a noble gas, an atom will achieve stability.
- When you have 2 electrons in the 1st shell, you have a duplet structure.
- When you have 8 electrons in the rest of the shells, you have an octet structure.
Chemical Bonds
There are 3 ways of forming chemical bonds between atoms:
- Ionic Bonding
- Covalent Bonding
- Metallic Bonding
1. Ionic Bonding
Ionic bonding is usually formed between metals and non-metals. These ionic bonds result from the transfer of electrons from metal atoms to non-metal atoms forming positive and negative ions (cations and anions). The electrical forces between these oppositely charged ions form strong ionic bonds.
Naming of ionic bonds:
[Name of cation][Name of anion]
e.g. Sodium Chloride
2. Covalent Bonding
Some atoms form bonds by sharing electrons to gain the electronic configuration of a noble gas (obtain a full valence shell). These bonds are known as covalent bonds.
A covalent bond is formed by the sharing of a pair of electrons between two atoms. Each atom contributes one electron to the bond. A covalent bond may have a single, double or triple covalent bond.
Generally, covalent bonds are formed between atoms of non-metal. Covalent bonds can be formed between atoms of same elements (O2, H2) or atoms of different elements (H2O, CO2). Compounds which contain covalent bonds are known as covalent compounds.
Single covalent bond - One pair of shared electrons between two atoms
Double covalent bond - Two pairs of shared electrons between two atoms
Triple covalent bond - Three pairs of shared electrons between two atoms
2. Covalent Bonding
Some atoms form bonds by sharing electrons to gain the electronic configuration of a noble gas (obtain a full valence shell). These bonds are known as covalent bonds.
A covalent bond is formed by the sharing of a pair of electrons between two atoms. Each atom contributes one electron to the bond. A covalent bond may have a single, double or triple covalent bond.
Generally, covalent bonds are formed between atoms of non-metal. Covalent bonds can be formed between atoms of same elements (O2, H2) or atoms of different elements (H2O, CO2). Compounds which contain covalent bonds are known as covalent compounds.
Single covalent bond - One pair of shared electrons between two atoms
Double covalent bond - Two pairs of shared electrons between two atoms
Triple covalent bond - Three pairs of shared electrons between two atoms
Making and breaking chemical bonds
When a chemical reaction occurs, one substance changes to another. This means that bonds in the reactants must first be broken and then new bonds must be made in the products. Breaking bonds involves pulling atoms apart and this requires energy. However, making bonds helps to make atoms and this gives out energy. So, bonk breaking is endothermic and bond making is exothermic.
The Periodic Table
The Periodic Table is arranged in order of Groups and Periods.
(a) Group
The groups in the Periodic Table are numbered from I to VII and then Group 0. Some of these groups have names:
Group I: Alkali Metals
Group II: Alkaline Earth Metals
Group VII: Halogens
Group 0: Noble Gases
Elements between Group II and III are known as transition metals or transition elements.
Elements in the same group have similar chemical properties and will undergo the same type of chemical reactions.
(b) Period
Each period is numbered. Elements in the 1st period will only have their 1st shell either partially or fully occupied with electrons.
Elements in the 2st period will have their 1st shell fully occupied with electrons, and their 2nd shell fully or partially equipped with electrons.
Periodic Table Overview
The periodic table shows all the elements arranged in order of increasing atomic number.
(i) Electronic structure
Down each group, the number of valence electrons is the same for each element and is equal to the group number. For example, in Group I elements, we have:
Li - 2.1
Na - 2.8.1
K - 2.8.8.1
As Group I elements are very reactive, and elements with similar electronic configurations (similar number of valence electrons) have similar chemical properties, elements in the same group have similar chemical properties.
(ii) Metals and non-metals
Across the period, the properties of elements change from metallic to non-metallic.
The elements in blue are metals, the elements in yellow are non-metals and the elements in purple are metalloids. Metalloids have some properties of metals and non-metals, thus they are neither metal nor non-metal.
Order of reactive metals:
Order of reactive metals:
Practically all the elements after Hydrogen do not react with acids to give hydrogen gas and are the least easily corroded.
Formation Of Ions
Formation of cations
When an atom loses one or more electrons, it becomes a positively charged particle called a cation.
In a Lithium atom, there are 3 protons and 3 electrons. In a lithium ion, there are 3 protons and 2 electrons. Therefore, the lithium ion carries an overall positive charge of 1+ and is written as Li+.
Formation of anions
When an atom gains one or more electrons, it becomes a negatively charged particle called an anion.
In a Fluorine atom, there are 9 protons and 9 electrons. In a Fluoride ion, there are 9 protons and 10 electrons. Therefore, the fluoride ion carries an over positive charge of 1- and is written as F-.
So why do atoms become ions?
Atoms need to become ions so that it can obtain a full valence shell and hence bond with other elements via ionic bonding. Ions can lose or gain up to 3 electrons to obtain a full valence shell. This also means that any atom with a valency of 4 can never become an ion.
Charges on the ions are related to the group number and number of valence electrons. Elements on the left side of the Periodic Table lose their valence electrons to form cations with charges corresponding to their group number, while elements on the right side of the periodic table gain electrons to form anions corresponding to their group number to fill their valence shells with 8 electrons.
Charges on the ions are related to the group number and number of valence electrons. Elements on the left side of the Periodic Table lose their valence electrons to form cations with charges corresponding to their group number, while elements on the right side of the periodic table gain electrons to form anions corresponding to their group number to fill their valence shells with 8 electrons.
Atomic Structure
What is an atom?
An atom is the smallest unit of an element, having the properties of that element.
Some fast facts on atoms:
- All atoms are built from just three particles - Protons, Neutrons and Electrons.
- The centre of an atom is called the nucleus and this contains the protons and neutrons. The nucleus takes up less than 1% of the volume of an atom.
- Protons and neutrons have virtually the same mass. The proton and neutron each have a relative mas of one.
- Protons have a positive charge, but neutrons are neutral.
- The other 99% of the atom is empty space occupied by moving electrons.
- These electrons have a mass of about 2000 times less that of a proton or neutron.
- Electrons have a negative charge, which cancels out the positive charge on the proton.
- Electrons move around very rapidly, and occupy different shells at different distances from the nucleus.
Now, each shell can hold a certain maximum number of electrons. The 1st shell can hold 2 electrons, the 2nd shell can hold 8 electrons while the 3rd shell can also hold 8 electrons. For elements after calcium, their third shell can hold up to 18 electrons.
The atomic number is the number of protons in an atom while the mass number is the number of protons AND the number of neutrons in an atom.
Some atoms come from the same element, but have different masses. These different atoms are called isotopes. Isotopes are atoms with the same atomic number, but with different mass numbers.
An example of an isotope is a chlorine isotope. Two chlorine isotopes, chlorine-35 and chlorine-37, both have 17 protons and electrons each, but chlorine-35 has 18 neutrons and chlorine-37 has 20 neutrons. Hence, they have different mass numbers, different masses and hence different physical properties from its other isotopes, as their properties depend on the mass of its atoms and molecules.
So, isotopes in a nutSHELL (pun intended):
Isotopes of the same element have the same number of protons, electrons, atomic number and chemical properties, but have different numbers of neutrons, mass numbers and physical properties.
Electronic Structure & Electronic Configuration
How to write electronic configuration:
Earlier we just saw that each shell can hold a maximum number of electrons. The 1st shell can hold 2 electrons, the 2nd shell can hold 8 electrons while the 3rd shell can also hold 8 electrons. For elements after calcium, their third shell can hold up to 18 electrons.
So now, for example, let's take a Nitrogen atom. A nitrogen atom has 7 electrons so it's electronic configuration can be written as 2.5
A Magnesium atom has 12 electrons, so its electronic configuration can be written as 2.8.2
An Argon atom has 18 electrons, so its electronic configuration can be written as 2.8.8
Valency
The shell which is the furthest away from the nucleus that is still occupied by electrons is called the valence shell, or the outer shell. The electrons in the valence shell are known as valence electrons, and in a chemical reaction, only these valence electrons are involved in the chemical bonding between these atoms.
The number of electrons an atom uses to form bonds is called its valency. So basically, an atom with 1, 2, 3 or 4 valence electrons would have a valency of 1, 2, 3 and 4 respectively, and an atom with 5, 6, 7 or 8 valence electrons would have a valency of 3, 2, 1 and 0 respectively.
First Science Practical: 2P1 - Properties of Acids and Alkalis
In this practical, we learnt about the properties of acids and alkalis, and experimented on how to tell the difference between acids and alkalis.
Here are the experiments we carried out on 6 substances, substance A, B, C, D, E and F.
1. Placed a drop of the substance onto a piece of i) blue litmus paper and ii) red litmus paper.
2. Tested the substances for electrical conductivity by putting an electrical conductivity tester into a small beaker containing the solution.
3. Added two drops of Universal Indicator into 2ml of the substance.
4. Added two drops of Methyl Orange into 2ml of the substance.
5. Added two drops of phenolphthalein into 2ml of the substance.
Here are the results:
1Ai) Turns red
1Aii) Stays red
2A) Dim
3A) Dark pink
4A) Red
5A) Colorless
1Bi) Turns red
1Bii) Stays red
2B) Very bright
3B) Red
4B) Dark Red
5B) Colorless
1Ci) Stays blue
1Cii) Stays red
2C) No light
3C) Green
4C) Dark orange
5C) Colorless
1Di) Turns slightly reddish
1Dii) Remains red
2D) Very bright
3D) Green
4D) Yellow
5D) Colorless
1Ei) Turns purple
1Eii) Turns dark blue
2E) Very bright
3E) Dark purple
4E) Orange
5E) Dark red
1Fi) Remains blue
1Fii) Turns blue
2F) Very dim
3F) Light purple
4F) Dark orange
5F) Dark red
The practical worksheet told us that methyl orange would be red at a pH of 1-4 and would change to yellow from 4-14, and phenolphthalein would be colorless at a pH of 1-9 and would become red at a pH of 9-14. Also, here are the colors that the universal indicator changes to at different pHs:
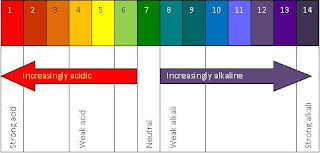
From this, we can see that substance A has a pH of 3, substance B has a pH of 1, substance C and D has a pH of 7, substance E has a pH of 14 and substance F has a pH of 11.
Thus, substance A is a weak acid, substance B is a strong acid, substance C and D is neutral, substance E is a strong alkali and substance F is a weak alkali.
Subscribe to:
Comments (Atom)


